Nov. 6, 2014
3 Bullets:
- ‘Big data’ cancer research has revealed a new spectrum of genetic mutations across tumors that need understanding.
- Existing methods for analyzing DNA defects in cancer are blind to how those mutations actually behave.
- ISB scientists developed a new approach using physics- and structure-based modeling to systematically assess the spectrum of mutations that arise in several gene regulatory proteins in cancer.
By Jake Valenzuela and Justin Ashworth
A significant challenge in cancer research is having the right tools and methods to analyze the myriad data generated by large-scale projects such as The Cancer Genome Atlas. Existing methods are too statistical and are blind to the way in which mutations actually affect protein function and biophysical mechanisms.
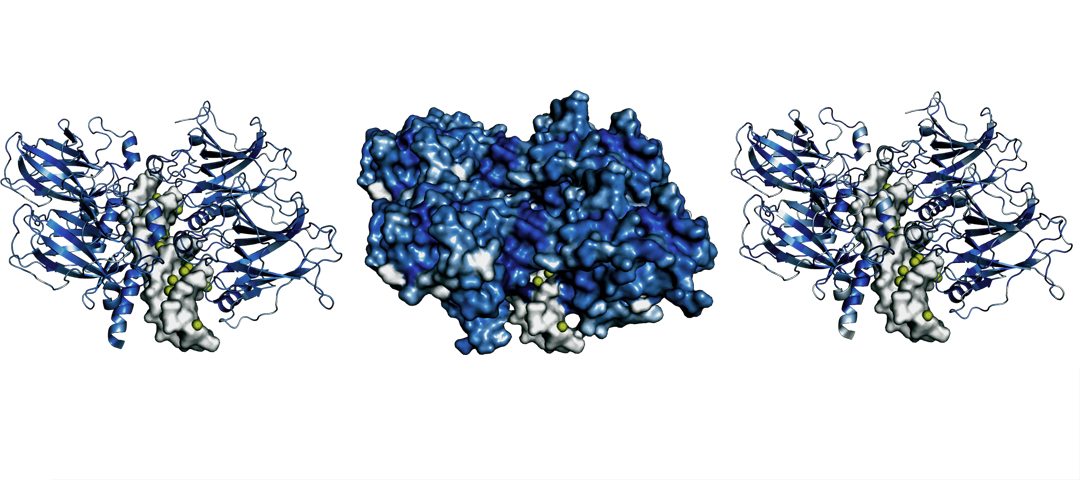
Researchers at Institute for Systems Biology have developed a new method that systematically investigates how small mutations in the DNA sequences that encode proteins result in structural and energetic changes that impact protein function. This study, a collaboration of the Baliga and Shmulevich labs, simulated the physics and energetics of protein structure and function in order to assess the impacts of a broad spectrum of protein mutations that have been observed in human cancers. Specifically, researchers considered mutations in proteins called transcription factors that regulate cellular function in order to predict and identify the putative effects of these mutations on gene expression and cell regulatory pathways.
Critical losses of protein function in gene regulatory proteins and tumor suppressors can lead to cellular dysregulation and the hallmarks of cancer. Using a physics-based approach to assess the impacts of mutations resulted in higher mechanistic accuracy in determining links between mutations in transcription factors and changes in gene expression. The method also illustrates a quantitative relationship between the relevant thermodynamic impacts of each unique protein mutation and its prevalence in cancerous tissues.
Integrating molecular biophysics and structure-based modeling into systems-based analyses of disease mutations will improve understanding of the molecular genetics of cancers and to interpret the complex data about mutations that are now available.
Title: Structure-based predictions broadly link transcription factor mutations to gene expression changes in cancers
Journal: Nucleic Acids Research
Authors: Justin Ashworth, Brady Bernard, Sheila Reynolds, Christopher L. Plaisier, Ilya Shmulevich, Nitin S. Baliga
Link: http://nar.oxfordjournals.org/content/early/2014/11/05/nar.gku1031.fullRead more about ISB’s work with The Cancer Genome Atlas project.


 isbscience.org/research/how-physics-and-thermodynamics-help-assess-dna-defects-in-cancer/
isbscience.org/research/how-physics-and-thermodynamics-help-assess-dna-defects-in-cancer/